IVQA Media-Fill Test Kits provide the tools to comply with USP Chapter <797> Media-Fill Test procedure standards on aseptic manipulation skills, including representative sterile microbial culture transfer and fill challenges. Our media-fill test kits are designed to assess correct technique and effective environmental control for the preparation of compounded sterile products.
Supporting compounding personnel in their aseptic techniques through our reliable media-fill testing solutions.
Finding the right kit for your application

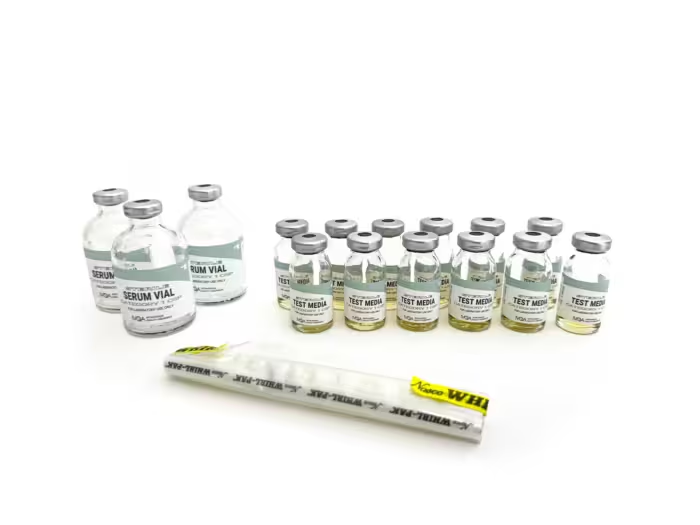
Category 1 CSP
Category 1 CSPs involve basic and rapid compounding activities with minimal contamination risk, including single-dose vial transfers, straightforward reconstitution of powdered medications, ampule opening and contents transfer, simple IV admixtures, syringe preparation for immediate use, and sterile-to-sterile product mixing in cases where procedures are direct and expedient.

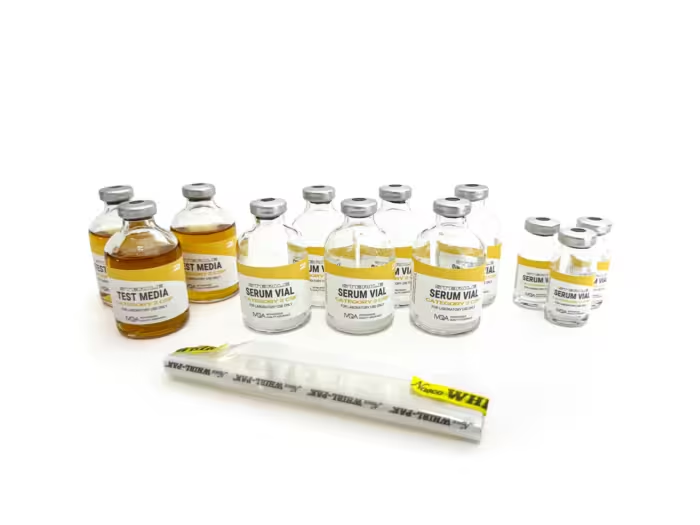
Category 2 CSP
Category 2 CSPs involve intermediate-level compounding tasks such as TPN compounding with precise multi-component mixing, multiple-step aseptic transfers, chemotherapy preparation with strict dosing, medication compounding for infusion pumps, batch preparations for several patients, and long-duration compounding processes, each demanding meticulous aseptic techniques to minimize contamination risks.

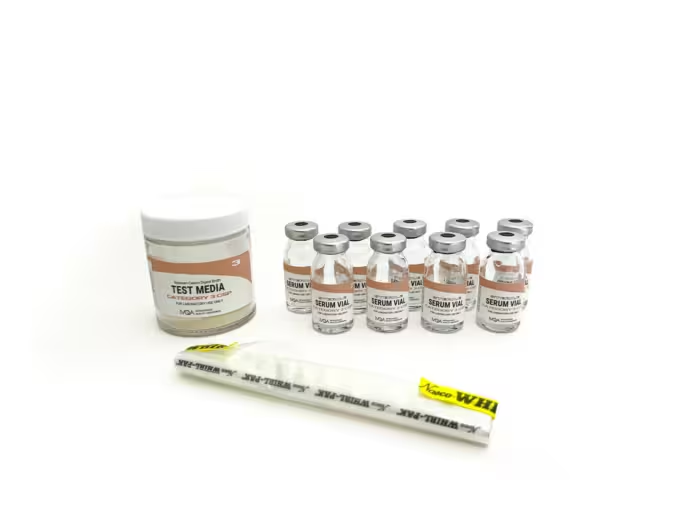
Category 3 CSP
Category 3 CSPs require advanced aseptic techniques due to their complexity and higher contamination risks, including the use of non-sterile ingredients and exposure to suboptimal air quality. Examples include bulk compounding for extended storage, incorporating non-sterile components for customized medication formulations, and utilizing sophisticated technologies for sensitive medications, all requiring rigorous sterilization measures post-compounding.