● Catalog No. MFT02
● Compliance with USP Chapter <797>
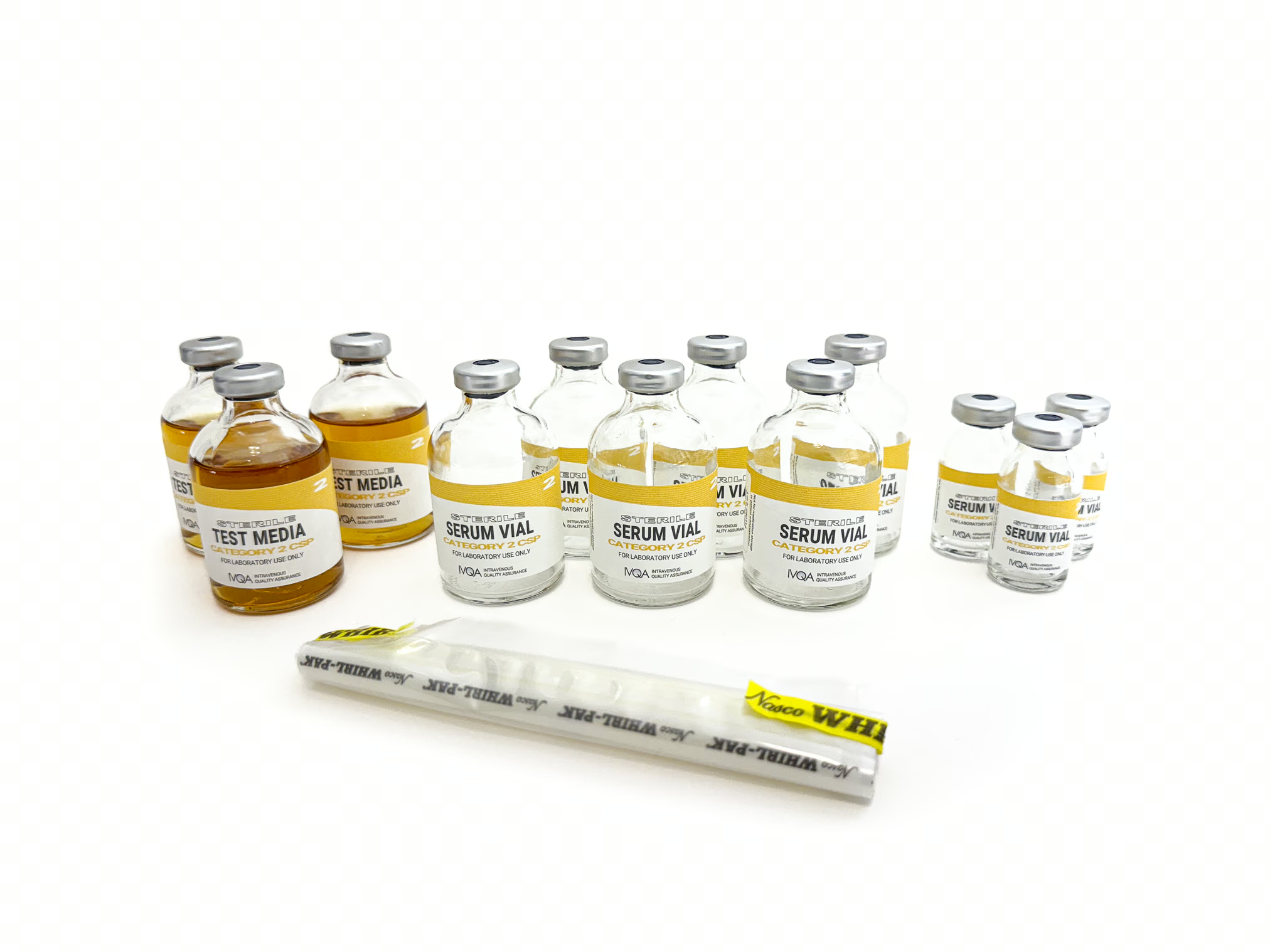
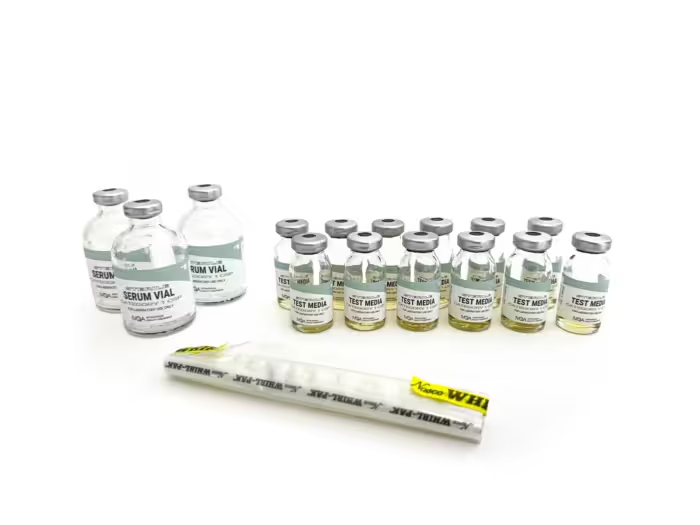
● Kit Contains:
◦ Sterile 50ml Test Media (Tryptic Soy Broth) – 3 vials
◦ Sterile 50ml Empty Serum Vials – 6 vials
◦ Sterile 10ml Empty Serum Vials – 3 vials
◦ Whirl-Pak® Bag – 1 bag
◦ Log Sheet
The Category 2 CSP Media-Fill Test Kit by IVQA validates compounding personnel’s aseptic technique and sterile compounding proficiency. Intended for simulating Category 2 compounded sterile preparations, it evaluates the risk of microbial contamination and assesses competency in aseptic techniques. Suitable for the initial assessment and every 6-month revalidation of aseptic manipulation competency, in accordance with USP Chapter <797> guidelines. This product is quality control tested for growth promotion and sterility.
USP Chapter <797> Category 2 CSP Guidelines
Description:
-
- Verifies aseptic techniques for preparations assigned a Beyond-Use Date (BUD) of more than 12 hours at controlled room temperature or beyond 24 hours when refrigerated.
- Designed for medium-complexity compounding activities that require enhanced environmental control and precision.
- Suitable for validating competencies in multi-step aseptic manipulations, complex reconstitutions, and handling of sensitive medications that have a elevated risk of contamination.
- All compounding activities must be performed in an ISO Class 5 or better air quality environment to minimize the risk of contamination.
Examples:
-
- Creating a nutrient mixture with multiple components (e.g., amino acids, vitamins, lipids) requiring precise measurements and multiple transfers.
- Processes involving multiple aseptic transfers from different containers, increasing contamination risk due to the number of manipulations.
- Compounding chemotherapy agents, which often involves mixing several drugs with precise dosing and handling hazardous materials.
- Compounding medications for infusion pumps, including multi-drug combinations and accurate reservoir filling.
- Preparing multiple doses of a sterile product in a single session, intended for use in multiple patients.
- Preparations that take a significant amount of time to complete, increasing the risk of contamination and requiring sustained aseptic technique.
- Compounding sensitive medications that have a high risk of microbial contamination or require high precision.
Initial Training and Competency:
-
- Before beginning independent compounding or having direct oversight of compounding personnel, individuals must successfully complete an initial aseptic manipulation competency evaluation.
- This initial evaluation includes visual observation, media-fill testing, gloved fingertip and thumb sampling on both hands, and surface sampling of the direct compounding area.
Frequency of Ongoing Competency Testing:
-
- Aseptic manipulation competency evaluations must occur at least every 6 months for personnel compounding Category 2 CSPs.
Test Procedure:
Within an ISO Class 5 air quality environment, arrange the vials into three sets, each containing one 50ml test media vial, two empty 50ml serum vials, and one empty 10ml serum vial.

Using a syringe equipped with a vented needle, transfer 25ml from the 50ml test media vial into each empty 50ml serum vials. Repeat this process for the remaining sets of vials. If your standard practice involves using IV transfer tubing sets with vented needles or spikes, you should opt for this transfer method instead.
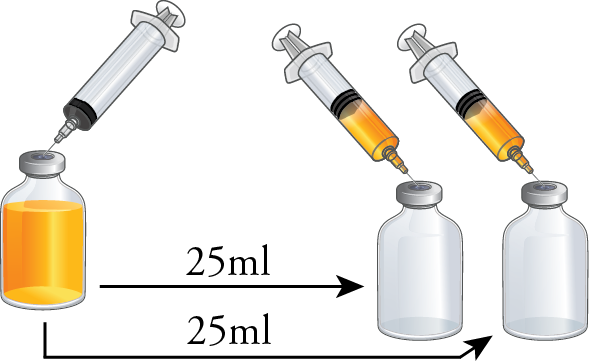
With the two 50ml vials now containing 25ml of test media each, perform four aseptic transfers of 5ml test media between both vials. Repeat this process for the remaining sets of vials.
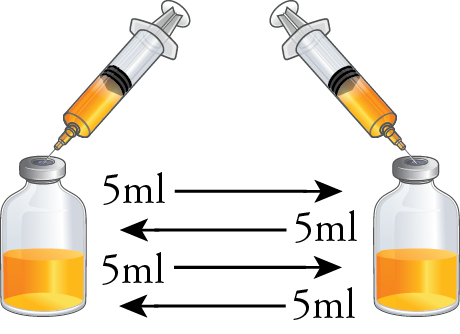
Aseptically transfer 5ml from both 50ml vials into the empty 10ml serum vial. Repeat this process for the remaining sets of vials. When completed, you should have three 10ml vials filled with 10ml of test media, one from each set.

Write your name and date of preparation on the three 10ml vial labels, one from each set. Then, aseptically apply a sterile adhesive seal to the rubber closures and place the sealed vials inside the provided Whirl-Pak® bag for transfer to the incubator.
Incubate the three 10ml vials at 20°–25°C (68°– 77°F) and 30°–35°C (86°– 95°F) for a minimum of 7 days at each temperature range to detect a broad spectrum of microorganisms. A total incubation period of at least 14 days is recommended to confirm the absence of microbial growth. Any signs of microbial growth, such as turbidity or precipitation, at any point during the incubation period, indicates a failure in the sterility test.
Once the test is initiated:
- Must be carried out to completion without interruptions.
- Performance conditions should mimic the usual work environment.
References:
-
- USP Chapter <797> guidelines can be found in the current version of the United States Pharmacopeia and National Formulary (USP–NF), which outlines the standards for sterile compounding procedures and quality assurance practices.
- Whirl-Pak® is a registered trademark of Nasco International, Inc.
| Weight | 2.0 lbs |
|---|---|
| Dimensions | 2 × 12 × 12 in |